Great idea!he just calls them " drippers" but is the same thing. It would be really good for a denitrator, all you would need is to split off a hose from your overflow and fit one of these, and you can add a denitrator without having to buy a pump.
These readings are not very different and may actually be within the accuracy of your measuring device (i.e. there could be no difference). Another possibility is that your tap water is being measured coming right out of an aerator, while your tank water has settled down.Ph
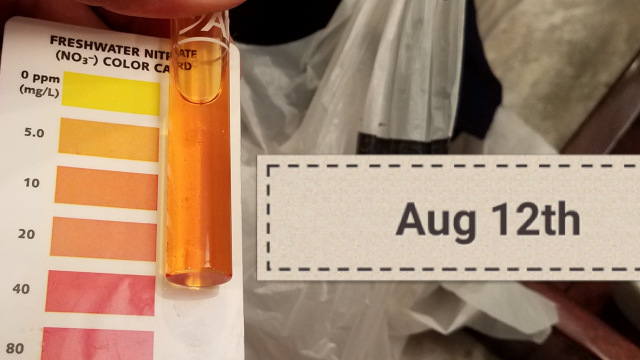
Tap 6.6 or 6.8
Tank 7.2
Under anoxic conditions, pH tends to fall due to an increase in the occurrence of free hydrogen molecules (H+) as redox reaction occur. Calcium carbonate does not have a direct role on redox reactions or denitrification. However, there can be an indirect influence via the relationship between hardness, alkalinity and pH in a particular tank. That is, if pH is maintained at a particular level due to high alkalinity, which might in turn be due to high calcium carbonate hardness, then the types of redox reactions that are likely to occur will be more predictable over time. Put another way, calcium carbonate doesn't help or hurt you with respect to biological denitrification in freshwater, but it might help keep your tank chemistry more stable. This is along the lines of whatActually, denitrifying bacteria do raise pH by increasing alkalinity. In nitrification, for every 1 mg of ammonia oxidized 7-8 mg alkalinity (as CaCO3) is removed from the water. In denitrication the opposite happens but not to the same extent. But I don't think that's the reason
Main concerns for sulfur systems that he mentioned is that when flow rate is too high and it isn't fully cycled it can leach sulfur and there is nothing to consume it which can be harmful to fish. Also without good discharge or aeration hydrogen can also poison your fish which is a big concern.
no worries about hydrogen in the air? I'm thinking of the Hindenburg, hydrogen is not your friend.
This is a REDOX Ladder:The type of hydrogen the person atHendre 's lfs was talking about was probably hydrogen sulfide.

Biological mediation of redox reactions are most likely to occur at the upper most levels (aerobes) because there is more energy to gain from the reaction. As you move down the ladder, these reactions are less likely to occur in a meaningful way in your fish tank. That said, if the flow through your "denitrate filter" is so slow that the ability to catalize the reduction of sulfate becomes advantageous from a competition stand point, then your filter will start to produce H2S. As you can see from the above, this is an extreme case and should be easily avoided by tweaking your denitrate filter flow-through rate until the output comes close to zero (as was suggested by
As for hydrogen, yes, it is in the form H2S (not H2 or H+). H2S is rapidly oxidized in the presence of oxygen, so I doubt it would have much of an effect on your fish so long as they are in a well aerated tank. H2S would be much more dangerous in the context of an deep substrate bed that has been anoxic for several weeks/months and is suddenly disturbed en-masse.