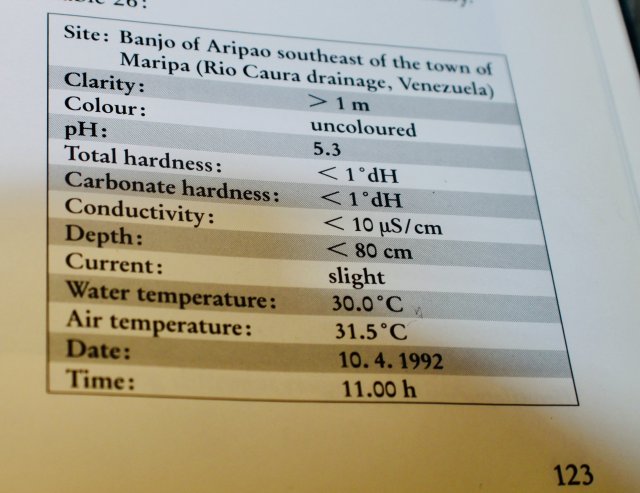
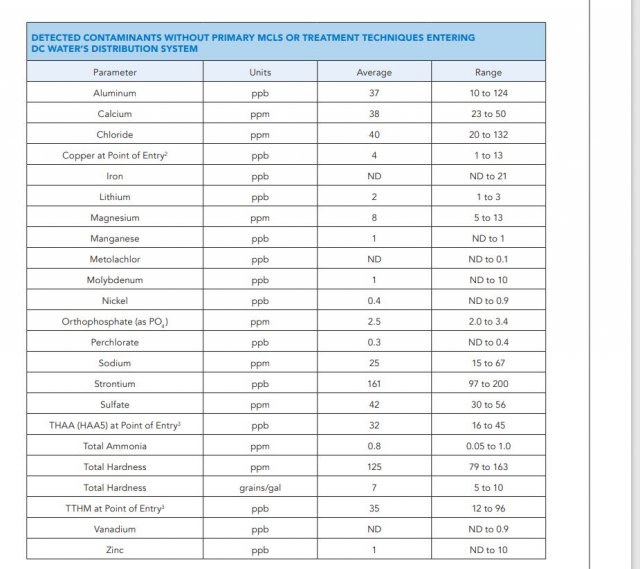
Ever since I grew up with fishkeeping these two terms have been interchanged and confusing...because of this I always thought ph = hardness. (alkaline hard, acidic soft)
Acidic, Neutral and Alkaline my understanding this has to do with ion charge?
Hardness is what, dissolved solids? I would wonder what neutral water would be like then. More recently I 've read the term grains.
Acidic, Neutral and Alkaline my understanding this has to do with ion charge?
Hardness is what, dissolved solids? I would wonder what neutral water would be like then. More recently I 've read the term grains.