Merrimack county, Warner, NHWhat city (or county if that's how water is distributed) and what state are you in?
Reverse Osmosis System
- Thread starter -603FiShHoBbIeSt-
- Start date
Below are 2 NH cities water quality reports, nitrate in both are negligible.
Both have water I consider fairly neutral pH, but low alkalinity, meaning a lack of buffering capacity. Because of the low buffering capacity fish urine can easily overwhelm a tank, create pH drops, which contribute to high nitrate.
To me this also means lots of water changes to maintain stability if a tank is in the least bit overstocked.
waterquality
2016 Water Quality Report.pdf
Both have water I consider fairly neutral pH, but low alkalinity, meaning a lack of buffering capacity. Because of the low buffering capacity fish urine can easily overwhelm a tank, create pH drops, which contribute to high nitrate.
To me this also means lots of water changes to maintain stability if a tank is in the least bit overstocked.
waterquality
2016 Water Quality Report.pdf
Well that could definitely be the awnswer to my high nitrate problemBelow are 2 NH cities water quality reports, nitrate in both are negligible.
Both have water I consider fairly neutral pH, but low alkalinity, meaning a lack of buffering capacity. Because of the low buffering capacity fish urine can easily overwhelm a tank, create pH drops, which contribute to high nitrate.
To me this also means lots of water changes to maintain stability if a tank is in the least bit overstocked.
waterquality
2016 Water Quality Report.pdf
Lol what?b
Most adult fish will tolerate thousands of ppm nitrate without harm. It's basically non-toxic except to the eggs and fry of certain species, mostly salmon/trout.
https://www.researchgate.net/public...oticus_L_in_recirculating_aquaculture_systems
500 mg/L NO3-N is 2,213 mg/L in NO3- (API test kit units).Abstract
Studies on chronic or acute toxicity of nitrogen species on fish in recirculating aquaculture systems (RAS) usually focused on adverse effects of total ammonia nitrogen (TAN: sum of NH3 + NH4+) and nitrite (), while underestimating the potential effects of high nitrate accumulation on growth and health status of fish. In our study, Nile tilapia (Oreochromis niloticus) were exposed to five different nitrate concentrations (0, 10, 100, 500 and 1000 mg L−1 -N) over 30 days. Growth parameters (feed conversion ratio (FCR), specific growth rate (SGR), hepatosomatic index (HSI)), blood samples (concentrations of haemoglobin, methaemoglobin, plasma /) and the histology of the gills were studied to evaluate growth and health status of the fish. At the highest nitrate concentration, the fish showed significantly reduced growth and impaired health status (SGR, FCR, plasma /, haemoglobin and methaemoglobin concentration), demonstrating that too high nitrate concentrations can negatively influence tilapia production in RAS. Here, we recommend not exceeding concentrations of 500 mg L−1 -N in juvenile tilapia culture to ensure an optimal health and growth status of the fish, as below that concentration no effects on the tilapia have been observed.
Until today, the uptake of nitrate is still poorly understood, mainly due to the fact that most tis- sues represent a barrier preventing the passage of the large hydrated nitrate ion. In their study on nitrate toxicity to African catfish (Clarias gariepinus), Schram, Roques, Abbink, et al. (2014) concluded that the integument of the fish forms a significant barrier to waterborne nitrate. As a consequence, alternative routes for nitrate uptake are limited and uptake via the gills seems most plausible with regard to the direct contact with the ambient water as well as the importance in osmoregulation and ion uptake (Hwang 2009). However, a low permeability for nitrate through the gills was discussed in trout (Stormer et al. 1996) and has been reported in freshwater crayfish (Jensen 1996).
I'm very familiar with that review. When it recommends 2 mg/L it's considering all life stages of invertebrates, amphibians, and fish. Early developmental stages of certain species are significantly more sensitive than others. It can be said that the food chain is only as strong as its weakest links so when recommending limits on nitrate levels in waterways, it should be low.
On the other hand, few aquarists rely on the food chain to feed their fish. Most of us are also not breeding cutthroat trout or coho salmon, whose eggs and fry are among the most sensitive.
Take a look at the actual data:

A 48-hr LC50 of 462 mg/L NO3-N for daphnia neonates? That's 2,045 mg/L NO3- (API units)!
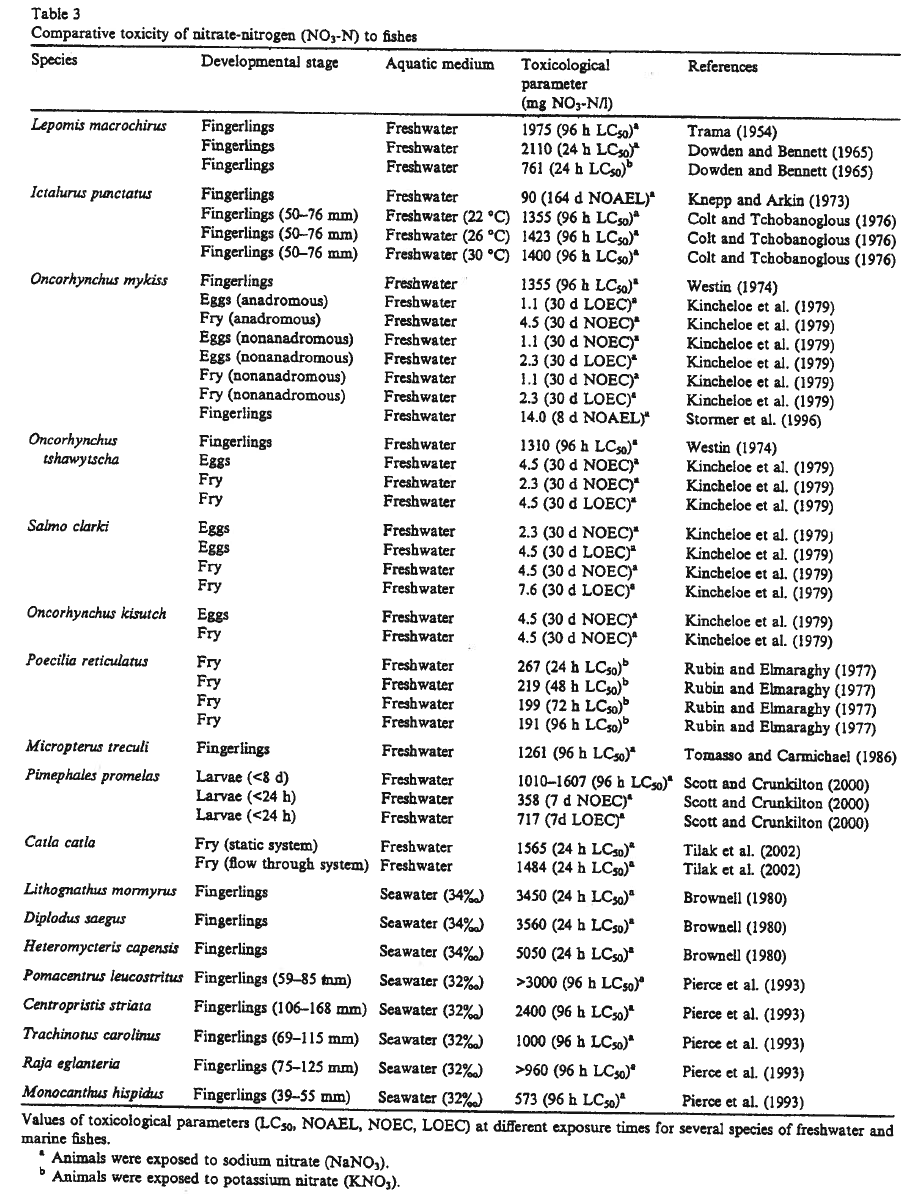
Fathead minnow larvae <24 hrs old have a 7-day NOEC of 358 mg/L NO3-N. That's a whopping 1,584 NO3-. Well, they're tough fish. What about coho salmon, one of the most sensitive fish species to nitrate (2.3 mg/L NO3-N)? Well, as fingerlings their 96-hr LC50 is 1,310 mg/L NO3-N or 5,800 mg/L NO3-.
The author of that article doesn't realize that all the scientific articles use NO3-N so when he (or she) is advocating for 20 ppm, it's really 89 ppm (API units). He also doesn't account for the typically vast difference in nitrate tolerance between life stages. Once a fish has an intact integument (e.g., has developed beyond fry), it's basically impermeable to nitrate.Is Nitrate Toxic? A Study of Nitrate Toxicity - Oscarfish.Com
Ammonia is toxic because NH3 can passively diffuse from the water through the gills if water levels are greater than blood levels. Ammonia is also constantly being produced in the fish. Ammonium can be exchanged for H+ at the gills but against a large concentration gradient that may become difficult. Ammonia is much more toxic than nitrate due to its permeability and the fact that fish are always building up levels in their blood. Nitrate is neither permeable to the gills nor produced internally.
Nitrite in the water is mistakenly uptaken by the Cl-/HCO3- exchanger in the gills as fish try to scavenge CL- from the water. Fish can actively concentrate blood nitrite up to 60X the level in water.
There is no such mechanism for nitrate uptake. The tilapia study tells us that the route for nitrate is incidental ingestion (and we know freshwater fish don't "drink" much). Nitrate is reduced to nitrite in the stomach and the fish suffer the same symptoms of nitrite toxicity. It takes a very high nitrate level for the ingestion of small amounts of water to result in nitrite toxicity.
Very interesting thank you for this perspective and informationI'm very familiar with that review. When it recommends 2 mg/L it's considering all life stages of invertebrates, amphibians, and fish. Early developmental stages of certain species are significantly more sensitive than others. It can be said that the food chain is only as strong as its weakest links so when recommending limits on nitrate levels in waterways, it should be low.
On the other hand, few aquarists rely on the food chain to feed their fish. Most of us are also not breeding cutthroat trout or coho salmon, whose eggs and fry are among the most sensitive.
Take a look at the actual data:

A 48-hr LC50 of 462 mg/L NO3-N for daphnia neonates? That's 2,045 mg/L NO3- (API units)!

Fathead minnow larvae <24 hrs old have a 7-day NOEC of 358 mg/L NO3-N. That's a whopping 1,584 NO3-. Well, they're tough fish. What about coho salmon, one of the most sensitive fish species to nitrate (2.3 mg/L NO3-N)? Well, as fingerlings their 96-hr LC50 is 1,310 mg/L NO3-N or 5,800 mg/L NO3-.
The author of that article doesn't realize that all the scientific articles use NO3-N so when he (or she) is advocating for 20 ppm, it's really 89 ppm (API units). He also doesn't account for the typically vast difference in nitrate tolerance between life stages. Once a fish has an intact integument (e.g., has developed beyond fry), it's basically impermeable to nitrate.
Ammonia is toxic because NH3 can passively diffuse from the water through the gills if water levels are greater than blood levels. Ammonia is also constantly being produced in the fish. Ammonium can be exchanged for H+ at the gills but against a large concentration gradient that may become difficult. Ammonia is much more toxic than nitrate due to its permeability and the fact that fish are always building up levels in their blood. Nitrate is neither permeable to the gills nor produced internally.
Nitrite in the water is mistakenly uptaken by the Cl-/HCO3- exchanger in the gills as fish try to scavenge CL- from the water. Fish can actively concentrate blood nitrite up to 60X the level in water.
There is no such mechanism for nitrate uptake. The tilapia study tells us that the route for nitrate is incidental ingestion (and we know freshwater fish don't "drink" much). Nitrate is reduced to nitrite in the stomach and the fish suffer the same symptoms of nitrite toxicity. It takes a very high nitrate level for the ingestion of small amounts of water to result in nitrite toxicity.
http://www.yorku.ca/spk/fishbiol09/FB09lecture11.pdf

Only nitrogenous wastes are consumed by nitrifiers therefore fish urine contributes very little towards the alkalinity consumed by the nitrification process.