Measuring Dissolved O2 In The Water?
- Thread starter Kittiee Katt
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Hello; Back in the 1970's I took a course called limnology. We studied ponds and streams. There was a test for dissolved O2. As I recall, I put a sample of the water in a container and had to immediately put in a chemical to "lock" the O2 because it would change if I did not. Then we hauled the samples back to a lab to finish up. It took a hour or so to get the water parameters in the lab. Another bit of outdated experience. I do think there are simpler ways now but have not had reason to keep up.
By the way, does anyone understand why the thread "the truth about bettas" was shut down? It seemed to be going well. I am curious if anyone was going to respond to my last long post. Dissolved O2 was a major part of that discussion. I hope my last post did not lead to the closure as I was trying to be humorous and did not think we were actually having an argument. Oh well, I have no say so about these things.
By the way, does anyone understand why the thread "the truth about bettas" was shut down? It seemed to be going well. I am curious if anyone was going to respond to my last long post. Dissolved O2 was a major part of that discussion. I hope my last post did not lead to the closure as I was trying to be humorous and did not think we were actually having an argument. Oh well, I have no say so about these things.
Sera (German) produces an O2 test kit, I bought mine on a whim, used it a few times, shows my O2 was fine, haven't lost fish so I guess it is OK. From memory cost about the same as other test kits, around $20. Haven't used it in a while, quite frankly don't know even where I put it. If you search the Sera site, I'm sure you'll find the info you want.
I've no idea why it was closed, I didn't realise it was. I didn't see it as an argument either.By the way, does anyone understand why the thread "the truth about bettas" was shut down?
Also thanks everyone who responded.
The kind of DO probes sold by FSci need to meet EPA, and state standards for certified lab use, and are rigorously tested for accuracy, thus the reflection in price.
By the way, does anyone understand why the thread "the truth about bettas" was shut down? It seemed to be going well. I am curious if anyone was going to respond to my last long post. Dissolved O2 was a major part of that discussion. I hope my last post did not lead to the closure as I was trying to be humorous and did not think we were actually having an argument. Oh well, I have no say so about these things.
If it was shut down, it seems to be "opened up" again
Hello; You are correct. I just made a post on the thread. I could not yesterday for some reason. My bad.If it was shut down, it seems to be "opened up" again
DO is measured in units of mg/l (or ppm, interchangeable units, 1:1) OR as a percentage value. The amount of O2 that can be dissolved in water is dependent on the temperature of the sample, so frequently a % is used to report the DO value.
There are test kits and meters for testing DO, as stated by others. The most important thing with DO accuracy is sample handling, as DO is a constant moving target. Leave a sample in a sealed jar, and bacteria will consume O2, or planktonic algae will produce O2. This is why, as stated by S skjl47 earlier, samples need to be "fixed" in the field if they will be tested at a later time. For this same reason, I prefer a direct reading meter for surface water sampling. This is more complicated if you are collecting samples at depth, for obvious reason... Here is a link to the LaMotte brand kit, and in the link there is a very detailed instructions manual that give you a good bit of information with recommendations for techniques and some of the science behind the methodology.
http://www.lamotte.com/en/aquarium-fish-farming/individual-test-kits/5860-01.html
As for DO meters, there are two primary types, galvanic and optical. Both are calibrated in the same way, by setting 100% concentration in a closed vessel, measuring the 02 level in moist air over water or a wet sponge.
Galvanic membrane probes have been around a lot longer, they have a membrane and electrolyte solution that must be replaced, which is a drawback. Also, galvanic sensors are consumptive, meaning that that consume oxygen from a sample as they measure the concentration. This has obvious drawbacks if you are working in a small sample, or if the membrane tip is resting on the bottom of a container, as it will consume the oxygen in the local area in which it is reading, and give a false low reading.
Here is a link to one particular galvanic DO probe that I use regularly:
http://www.wtw.de/us/produkte/labor/dissolved-oxygen/portable-meters.html
Optical DO probes use luminescent technology to measure O2 concentration. Essentially, an LED light replaces the membrane and electrolyte of the galvanic meters. These units require less maintenance, and the primary advantage is that they do not consume the O2 from the sample as they measure. There are many brands of these out there, and from my experience, they all perform similarly.
Here is a link to the one that I am currently using:
http://www.vernier.com/products/sensors/dissolved-oxygen-probes/
All that being said, the LAST thing that I would suggest spending money on in terms of water quality measurement is anything to do with DO! If you have ANY agitation of the surface of the water, or ANY form of aeration (which also leads to surface agitation...) then you will find that your DO values in your home aquarium will be consistently at or near 100%. Measuring DO in natural water is much more important, whether there has been a chemical contamination that limits DO, or simply an excess of respiration from bacterial bloom, of if you are investigating benthic zones of lakes or ponds where decomposition exceeds photosynthesis. Worrying about the level of oxygen available to your fish makes about as much sense as worrying about the level of oxygen available to YOU! Yea, if you close yourself in a box (or seal the surface of an aquarium by excluding all surface agitation), you will have issues. If you want to spend money on testing equipment, we can talk about pH meters, ion selective electrodes for ammonium and nitrate, conductivity meters, or even simply higher quality test kits than the standard API "master" kit.
While typing this, I have been simultaneously monitoring the pH in my angel tank, which I am in the process of boosting from the horrific level of 3.8 through the addition of calcite... it is up to about 4.25 at the moment, took a pretty big jump initially, but is gradually climbing now as the calcite slowly dissolves. I wish I had thought to plug in the conductivity meter to record that change a the same time...
There are test kits and meters for testing DO, as stated by others. The most important thing with DO accuracy is sample handling, as DO is a constant moving target. Leave a sample in a sealed jar, and bacteria will consume O2, or planktonic algae will produce O2. This is why, as stated by S skjl47 earlier, samples need to be "fixed" in the field if they will be tested at a later time. For this same reason, I prefer a direct reading meter for surface water sampling. This is more complicated if you are collecting samples at depth, for obvious reason... Here is a link to the LaMotte brand kit, and in the link there is a very detailed instructions manual that give you a good bit of information with recommendations for techniques and some of the science behind the methodology.
http://www.lamotte.com/en/aquarium-fish-farming/individual-test-kits/5860-01.html
As for DO meters, there are two primary types, galvanic and optical. Both are calibrated in the same way, by setting 100% concentration in a closed vessel, measuring the 02 level in moist air over water or a wet sponge.
Galvanic membrane probes have been around a lot longer, they have a membrane and electrolyte solution that must be replaced, which is a drawback. Also, galvanic sensors are consumptive, meaning that that consume oxygen from a sample as they measure the concentration. This has obvious drawbacks if you are working in a small sample, or if the membrane tip is resting on the bottom of a container, as it will consume the oxygen in the local area in which it is reading, and give a false low reading.
Here is a link to one particular galvanic DO probe that I use regularly:
http://www.wtw.de/us/produkte/labor/dissolved-oxygen/portable-meters.html
Optical DO probes use luminescent technology to measure O2 concentration. Essentially, an LED light replaces the membrane and electrolyte of the galvanic meters. These units require less maintenance, and the primary advantage is that they do not consume the O2 from the sample as they measure. There are many brands of these out there, and from my experience, they all perform similarly.
Here is a link to the one that I am currently using:
http://www.vernier.com/products/sensors/dissolved-oxygen-probes/
All that being said, the LAST thing that I would suggest spending money on in terms of water quality measurement is anything to do with DO! If you have ANY agitation of the surface of the water, or ANY form of aeration (which also leads to surface agitation...) then you will find that your DO values in your home aquarium will be consistently at or near 100%. Measuring DO in natural water is much more important, whether there has been a chemical contamination that limits DO, or simply an excess of respiration from bacterial bloom, of if you are investigating benthic zones of lakes or ponds where decomposition exceeds photosynthesis. Worrying about the level of oxygen available to your fish makes about as much sense as worrying about the level of oxygen available to YOU! Yea, if you close yourself in a box (or seal the surface of an aquarium by excluding all surface agitation), you will have issues. If you want to spend money on testing equipment, we can talk about pH meters, ion selective electrodes for ammonium and nitrate, conductivity meters, or even simply higher quality test kits than the standard API "master" kit.
While typing this, I have been simultaneously monitoring the pH in my angel tank, which I am in the process of boosting from the horrific level of 3.8 through the addition of calcite... it is up to about 4.25 at the moment, took a pretty big jump initially, but is gradually climbing now as the calcite slowly dissolves. I wish I had thought to plug in the conductivity meter to record that change a the same time...

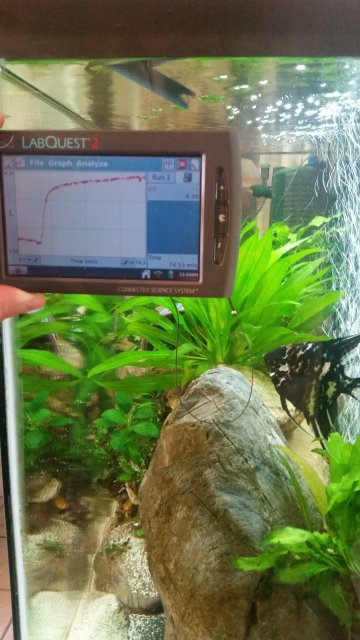
Okay, here are a few photos that are more pertinent to the thread...
These measurements were taken with the same meter, probes, and calibration only a few minutes apart.
As you can see, the trout tank is 57.1*F, and 99.5% DO is approximately 9.98 mg/l DO.
Compare this to the oscar tank, at 81.3*F, and 100% DO is approximately 7.54 mgl/l DO.
Although both tanks are at or near 100% DO, the warm water can only hold about 75% of the concentration of dissolved oxygen in the colder water. This is only of the biggest reasons that obligate cold water species are obligate cold water species - it is not the temperature directly that they cannot tolerate, it is the high concentration of oxygen in the water that they require.


I like how Spooky (the oscar) is clearly investigating the DO probe in the left picture... such curious fish, those oscars...
These measurements were taken with the same meter, probes, and calibration only a few minutes apart.
As you can see, the trout tank is 57.1*F, and 99.5% DO is approximately 9.98 mg/l DO.
Compare this to the oscar tank, at 81.3*F, and 100% DO is approximately 7.54 mgl/l DO.
Although both tanks are at or near 100% DO, the warm water can only hold about 75% of the concentration of dissolved oxygen in the colder water. This is only of the biggest reasons that obligate cold water species are obligate cold water species - it is not the temperature directly that they cannot tolerate, it is the high concentration of oxygen in the water that they require.


I like how Spooky (the oscar) is clearly investigating the DO probe in the left picture... such curious fish, those oscars...
If you want to spend money on testing equipment, we can talk about pH meters, ion selective electrodes for ammonium and nitrate, conductivity meters, or even simply higher quality test kits than the standard API "master" kit.
I'm happy to talk about all this, if you're willing to explain it all and answer all my questions.
I'm just curious as to what's out there in the hobby, the more I can learn the better imo.


