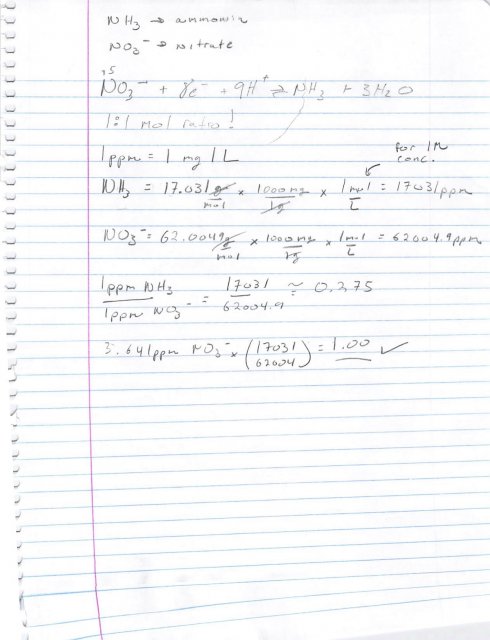Does anybody have a idea, on ammonia ppm ratio to nitrates after completing the nitrogen cycle?
Thanks,
Thanks,
Using atomic mass, 1 ppm of ammonia will become 3.641 ppm of nitrates. That's assuming the ammonia is fully converted to nitrate.
This is because ammonia is NH3, nitrate is NO4, and the atomic masses are N (14.00643), H (1.00784), & O (15.999.) The mass of ammonia is 17.03 and of nitrate is 62.003.