Water Quality
- Thread starter jaws7777
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
J. H. said:He probably has coral or shills or bones some sort of buffer in there. Or maybe the fancy denitrator bacteria raise the ph instead of lowering it.
Actually, denitrifying bacteria do raise pH by increasing alkalinity. In nitrification, for every 1 mg of ammonia oxidized 7-8 mg alkalinity (as CaCO3) is removed from the water. In denitrication the opposite happens. But I don't think this is the reason J jaws7777 tap pH is lower than his tank pH.
Hendre said:I have very soft water so why the high PH I'm not sure ... Mine comes in 9.0 and settles at 7.5, on that note I need an ageing barrel
My water is similar. It exits the tap around 8.6 and settles around 7.45 after aging.
The pH of water strait from the tap will rise or fall after aging/aerating in a cup, bucket or aging tank for ~24 hours. This is because CO2 levels differ between the water underground or in distribution pipes and between water at the surface. Water with excess CO2 will exit the tap at a lower pH and rise to higher pH after ~24 hours aging/aerating for ~24 hours. Water with relative lack of CO2 will exit the tap at a higher pH and fall to a lower pH after aging/aerating for ~24 hours.
This is separate from what happens in a tank. In a tank, changes in pH are due to alkalinity, typically KH. Acids from driftwood, leaves, nitrification, etc. erode KH; pH falls. Coral, aragonite, limestone, bicarbonate increase KH; pH falls.
Do you know you water's KH
EDIT: Wrong thread. There's no delete even within 10 minutes?
Actually, denitrifying bacteria do raise pH by increasing alkalinity. In nitrification, for every 1 mg of ammonia oxidized 7-8 mg alkalinity (as CaCO3) is removed from the water. In denitrication the opposite happens. But I don't think this is the reason J jaws7777 tap pH is lower than his tank pH.
My water is similar. It exits the tap around 8.6 and settles around 7.45 after aging.
The pH of water strait from the tap will rise or fall after aging/aerating in a cup, bucket or aging tank for ~24 hours. This is because CO2 levels differ between the water underground or in distribution pipes and between water at the surface. Water with excess CO2 will exit the tap at a lower pH and rise to higher pH after ~24 hours aging/aerating for ~24 hours. Water with relative lack of CO2 will exit the tap at a higher pH and fall to a lower pH after aging/aerating for ~24 hours.
This is separate from what happens in a tank. In a tank, changes in pH are due to alkalinity, typically KH. Acids from driftwood, leaves, nitrification, etc. erode KH; pH falls. Coral, aragonite, limestone, bicarbonate increase KH; pH falls.
Do you know you water's KHHendre ?
EDIT: Wrong thread. There's no delete even within 10 minutes?
Mike Lmaooo.... i dont have to be smart as long as im hangin with guys like you
I think i can confidently say this method is working.
Taken sat night after wc.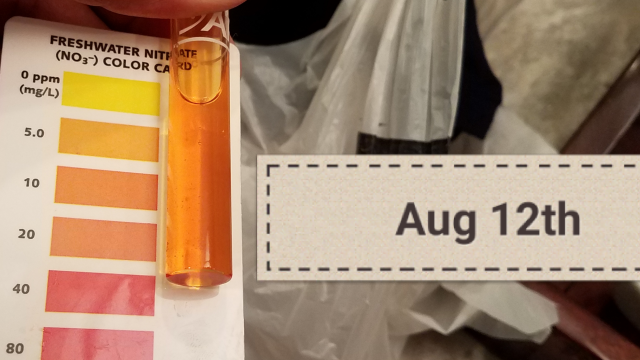
Last night i fed tilapia and tested this morning. Nitrates are between 0 and 5ppm.
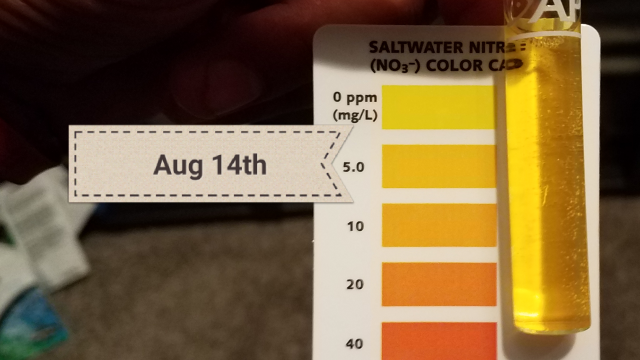
We still need to figure out why kno4tes filter cycled faster than mine so that future builds can be more successful.
Nitrates arent the only toxin we are removing with water changes but are probably the easiest to measure so i absolutely plan on conducting weekly wc's
Taken sat night after wc.

Last night i fed tilapia and tested this morning. Nitrates are between 0 and 5ppm.

We still need to figure out why kno4tes filter cycled faster than mine so that future builds can be more successful.
Nitrates arent the only toxin we are removing with water changes but are probably the easiest to measure so i absolutely plan on conducting weekly wc's
So somewhere between 10 and 20 down to 5 to 10. That's awesome! I'd guess maybe o2 level? Yours maybe slightly higher retarding the anaerobic bacteria growth.I think i can confidently say this method is working.
Taken sat night after wc. View attachment 1268333
Last night i fed tilapia and tested this morning. Nitrates are between 0 and 5ppm.
View attachment 1268334
We still need to figure out why kno4tes filter cycled faster than mine so that future builds can be more successful.
Nitrates arent the only toxin we are removing with water changes but are probably the easiest to measure so i absolutely plan on conducting weekly wc's
I accidently used the salt test card. Its more like 0 to 5 ppm. Closer to 0 will post another pic laterSo somewhere between 10 and 20 down to 5 to 10. That's awesome! I'd guess maybe o2 level? Yours maybe slightly higher retarding the anaerobic bacteria growth.


