I just got a fish aquarium and my ammonia levels are high. What do I do??
- Thread starter Jim bean 20055
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Yes the small aquarium has fish. I will add some
.25 ppm= 1/4 of a millionth
so 18,927/250,000 = 0.075 x 100 = 7.5708 x 2 = 15.14 which rounds to 15
Someone let me know if my math is correct
If it is I'm adding 15 grams of salt to my 5 gal aquarium
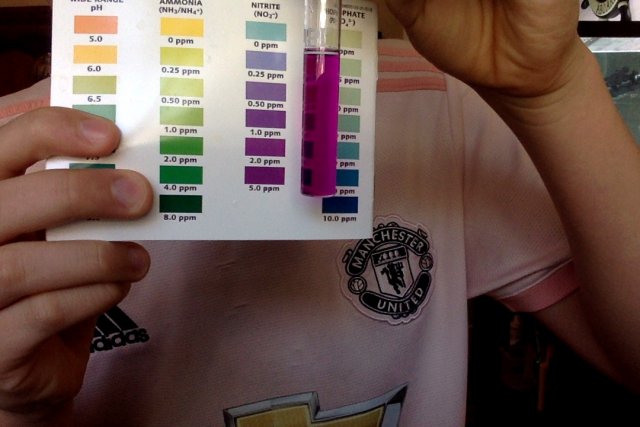
This is my nitrite testing from right now for my 60 gal aquarium
5 gal= 18,927 grams of waterNitrite must be detoxified. It's harmful at any concentration, unlike ammonia.
60 gal = 227,000 gram of water
1 ppm = one millionth
so 227,000 / 1,000,000 = 0.227 gram of nitrite are present in your aquarium.
For the sake of our rough estimation, nitrite NO2 and table salt NaCl molecular masses are not significantly different, so we will just continue working with weights in grams and not bother with the moles.
One must have table salt aka sodium chloride at 100x excess to detox the nitrite, so:
0.227 x 100 = 22.7 gram must be added to detox 1 ppm nitrite in a 60 gal tank
Since NaCl molecular mass is a bit heavier than nitrite and since NaCl is rather benign, I'd go with a 1.5x-2x excess by weight, that is, I'd add 45 gram of table salt to a 60 gal tank to detox 1 ppm of nitrite.
Keep testing for nitrite and remember to replenish table salt when you do a WC. For instance, if you do a 50% water change, you will have removed 50% of table salt, so must add 50% back, that is 23 gram.
You can use kitchen scale to weight out the salt. Make sure the salt you are using is (almost) pure, like 99%+ sodium chloride without any additives, water, etc. The salt used for water softeners is good.
.25 ppm= 1/4 of a millionth
so 18,927/250,000 = 0.075 x 100 = 7.5708 x 2 = 15.14 which rounds to 15
Someone let me know if my math is correct
If it is I'm adding 15 grams of salt to my 5 gal aquarium

This is my nitrite testing from right now for my 60 gal aquarium
Your nitrite result shows your tank is only 1/2 to 2/3 cycled.
That amount of nitrite should be considered toxic.
It means you (so far) do not have a sufficient population of beneficial bacteria to detoxify your tank water
Until nitrite is 0 and you get some "nitrate", the tank is not cycled.
That amount of nitrite should be considered toxic.
It means you (so far) do not have a sufficient population of beneficial bacteria to detoxify your tank water
Until nitrite is 0 and you get some "nitrate", the tank is not cycled.
1 tablespoon without heap is roughly 15 gramSo without a scale, it is roughly 1 tablespoon of fine salt per 20g of tank water for 1ppm of nitrite. (more than 1x excess by weight)
20 gal x 3 = 60 gal
15 gram x 3 = 45 gram, just like I recommended.
5 gal= 18,927 grams of water
.25 ppm= 1/4 of a millionth
so 18,927/250,000 = 0.075 x 100 = 7.5708 x 2 = 15.14 which rounds to 15
Someone let me know if my math is correct
If it is I'm adding 15 grams of salt to my 5 gal aquarium
No.
1/4 of one millionth = 1 / 4,000,000
May I please beg you to not post multiple posts in quick succession. You have 15 min to edit your post via the "Edit" button to add to it or to modify it. Please use them to the fullest. The way you post (like in a cell phone texting) makes everyone scroll 2x-3x more than we have to, because in the span of 5 minutes you post from 2 to 5 posts.
Haven't you noticed how your multiple consecutive posts have been combined above? I can't keep up with your fire speed. It will read too much nicer than a bunch of one-two worders or one liners.
Ok I wont post so much. Im just confused on how to dose my 5 gallon aquarium with salt. You said 1 ppm is 1,000,000 so i was thinking .25=1/4 would be 250,000. Im probably wrong. Sorry for posting so much.No.
1/4 of one millionth = 1 / 4,000,000
May I please beg you to not post multiple posts in quick succession. You have 15 min to edit your post via the "Edit" button to add to it or to modify it. Please use them to the fullest. The way you post (like in a cell phone texting) makes everyone scroll 2x-3x more than we have to, because in the span of 5 minutes you post from 2 to 5 posts.
Haven't you noticed how your multiple consecutive posts have been combined above? I can't keep up with your fire speed. It will read too much nicer than a bunch of one-two worders or one liners.
No, you are getting me wrong. You can post as much and more, much more, no problem. Just compile your posts. Use the EDIT button if you have to, in the first 15 minutes. Refrain from posting 5 posts one minute apart each but roll all these short posts into one bigger post. That's all. We welcome all your questions and reactions, etc.
The rest of your math is right. I only pointed out one arithmetic error.
1 ppm is not 1,000,000 but the reverse of it, that is 1 over 1,000,000.
If 1 ppm is one millionth, the 1/4 ppm must be smaller, and is indeed 4 times smaller or one 4 millionth.
The rest of your math is right. I only pointed out one arithmetic error.
1 ppm is not 1,000,000 but the reverse of it, that is 1 over 1,000,000.
If 1 ppm is one millionth, the 1/4 ppm must be smaller, and is indeed 4 times smaller or one 4 millionth.
ok let me try again so
5 gal- 18927 grams of water
.25 ppm- 4,000,000
18927/4 million=.0047 x 100 = .47 x 2 = .9 im so confused. I think i interpreted this wrong. So the math i did was right so I should be dosing 15 grams of salt because I don't think it should be 1 gram of salt. Unless I'm wrong and I do dose 1 gram of salt.
5 gal- 18927 grams of water
.25 ppm- 4,000,000
18927/4 million=.0047 x 100 = .47 x 2 = .9 im so confused. I think i interpreted this wrong. So the math i did was right so I should be dosing 15 grams of salt because I don't think it should be 1 gram of salt. Unless I'm wrong and I do dose 1 gram of salt.
5 gal = ~20,000 gram of water
0.25 ppm = one over 4 million
so 20,000 / 4,000,000 = 0.005 gram of nitrite are present in your aquarium.
For the sake of our rough estimation, nitrite NO2 and table salt NaCl molecular masses are not significantly different, so we will just continue working with weights in grams and not bother with the moles.
One must have table salt aka sodium chloride at 100x excess to detox the nitrite, so:
0.005 x 100 = 0.5 gram must be added to detox 0.25 ppm nitrite in a 5 gal tank
0.5 x 2 for safety = 1 gram
Thus, your latest calculation is correct. Only 1 gram.
***Sorry, when I said the rest of your math is right, I meant if you used the right number for 0.25 ppm, you would have gotten the right answer. Since you made an error early on, the answer came out wrong, although the rest of the calculation contained no arithmetic errors. Is this clearer?
***An easier way would be:
5 gal is 12 times smaller than 60 gal
0.25 ppm is 4 times smaller than 1 ppm
hence the amount of nitrite is 12 x 4 = 48 times smaller.
You needed 45 gram of salt for the 60 gal 1 ppm scenario. Hence 45 gram / 48 = 1 gram for the 5 gal 0.25 ppm scenario.
0.25 ppm = one over 4 million
so 20,000 / 4,000,000 = 0.005 gram of nitrite are present in your aquarium.
For the sake of our rough estimation, nitrite NO2 and table salt NaCl molecular masses are not significantly different, so we will just continue working with weights in grams and not bother with the moles.
One must have table salt aka sodium chloride at 100x excess to detox the nitrite, so:
0.005 x 100 = 0.5 gram must be added to detox 0.25 ppm nitrite in a 5 gal tank
0.5 x 2 for safety = 1 gram
Thus, your latest calculation is correct. Only 1 gram.
***Sorry, when I said the rest of your math is right, I meant if you used the right number for 0.25 ppm, you would have gotten the right answer. Since you made an error early on, the answer came out wrong, although the rest of the calculation contained no arithmetic errors. Is this clearer?
***An easier way would be:
5 gal is 12 times smaller than 60 gal
0.25 ppm is 4 times smaller than 1 ppm
hence the amount of nitrite is 12 x 4 = 48 times smaller.
You needed 45 gram of salt for the 60 gal 1 ppm scenario. Hence 45 gram / 48 = 1 gram for the 5 gal 0.25 ppm scenario.
Last edited:


